MTEL Chemistry (67) Practice Tests & Test Prep by Exam Edge
** Sample images, content may not apply to your exam **
Are you ready for advancement? The MTEL Chemistry exam, administered by one of the most respected qualifying organizations in the field, is a pivotal opportunity to demonstrate your knowledge and skills. Set yourself up for exam success with practice tests from Exam Edge.
Our MTEL Chemistry practice tests are designed to help you master both the subject matter and the art of test-taking. Created to mimic the length, difficulty, and feel of the real exam, our tests feature:
- Instant access to your online practice tests
- Unlimited Test Access - available 24/7
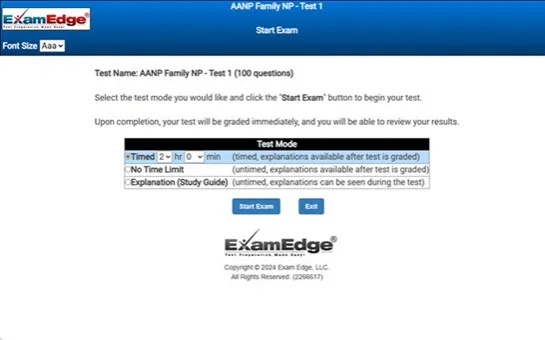
- Timed, untimed, and "study guide" modes
- Detailed explanations for each question
- 15 online practice exams with 100 unique questions per exam
- 1,500 unique questions, if you purchase all 15 exams
Start your exam prep today with an Exam Edge MTEL Chemistry practice test!
Not ready to purchase our complete practice tests yet? Start with a MTEL Chemistry FREE Practice Test first!

"My favorite thing about ExamEdge's online practice test is that they mimicked the actual exam. I walked into the exam feeling confident I knew the material and walked out knowing my time studying with Exam Edge was well worth the effort."
MTEL Chemistry (67) Shortcuts
General Exam Info
Exam Topics
Features
Study Plan Tips
Test Reviews
Why Exam Edge?
FAQ
FREE Practice Test
Pass Your MTEL Chemistry Certification Exam with Exam Edge
Are you nervous about your upcoming MTEL Chemistry certification exam? Look no further, Exam Edge is here to help you achieve your goal! Our world-class online practice certification tests are designed to provide you with the knowledge and confidence you need to pass your MTEL Chemistry exam on your first try!Key Facts on Exam Edge's online practice tests for your MTEL Chemistry Exam.
- 15 Online Practice Exams: With Exam Edge, you'll have access to 15 practice tests available, each with 100 questions, to help you thoroughly prepare for your MTEL Chemistry (67) exam.
- Instant Access: Get started right away with instant access to your online practice exams. No waiting or software installation required!
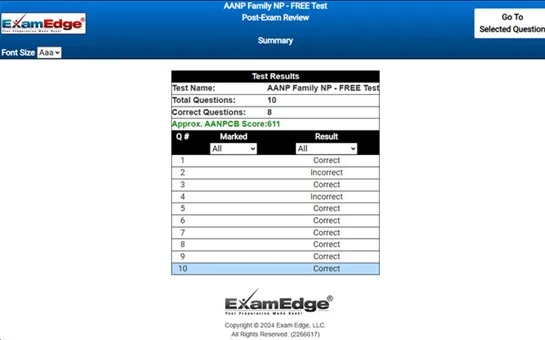
- Detailed Explanations: Each question is accompanied by a detailed explanation, so you'll fully understand the reasoning behind the correct answer and improve your knowledge.
- Take Each Exam 4 Times: You can take each of our practice exams up to 4 times, giving you plenty of opportunities to review and master the material.
- Web-Based: Our practice tests are available 24/7 at your convenience, and because they are web-based, there is no software to install.
- Psychological advantage: Exam Edge practice tests will allow you to walk into your test day with confidence.
Exam Edge practice tests are a valuable tool for helping you prepare for the Massachusetts Tests for Educator Licensure (67) certification exam. The practice tests provide a realistic simulation of the actual exam, allowing you to become familiar with the format, style, and types of questions you will encounter on the actual test. Certification exams don't just measure what you know -- they are also a test of how well you perform under pressure. The right type of test preparation helps you familiarize yourself not only with the material you're being tested on, but also the format of the test, so you feel less anxiety on test day.
MTEL Chemistry - General Exam Info Sample Questions
|
|
MTEL Chemistry (67) Exam Prep
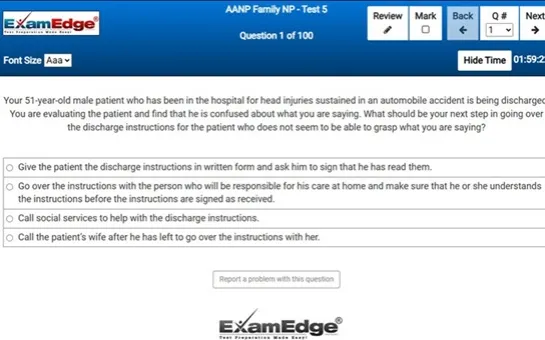
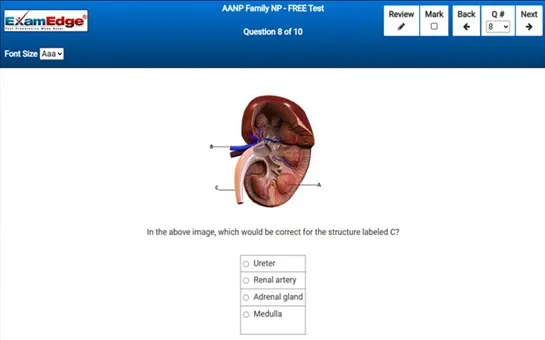
Our comprehensive MTEL Chemistry practice tests are designed to mimic the actual exam. You will gain an understanding of the types of questions and information you will encounter when you take your Massachusetts Tests for Educator Licensure Certification Exam. Our MTEL Chemistry Practice Tests allow you to review your answers and identify areas of improvement so you will be fully prepared for the upcoming exam and walk out of the test feeling confident in your results.
How to Prepare for the MTEL Chemistry Exam
So, you've decided to pursue your MTEL Chemistry certification. Not sure what comes next? Follow these steps to register for the exam, craft an effective study plan, and go into test day feeling confident.
Step 1: Check Eligibility and Apply for MTEL Chemistry
Start by researching the testing agency or credentialing organization and the different exams they offer for your field. Before you register for your exam, make sure that the MTEL Chemistry exam is the right match for your education, experience, and career goals.
Then, check whether you meet the requirements for taking the MTEL Chemistry exam. You can find eligibility information on the Massachusetts Tests for Educator Licensure website: Massachusetts Tests for Educator Licensure (MTEL). Once you have determined that you meet the qualifications or have completed the appropriate prerequisites, you can register with the organization and apply to take the MTEL Chemistry exam.
Step 2: Schedule the MTEL Chemistry
Once you have registered, you are ready to schedule your exam! The MTEL Chemistry exam is offered at various times throughout the year and at various locations across the United States. You can use the Massachusetts Tests for Educator Licensure website to find a testing center near you and choose a date and time that suits your availability.
When you schedule your MTEL Chemistry exam, consider how much time you want to study and prepare. Choose a test date that gives you plenty of time to create a study plan, thoroughly review the material, and take several practice tests so that you can go into test day feeling confident and ready. Be sure to schedule your exam well in advance to secure your preferred date and time.
Step 3: Study and Practice for the MTEL Chemistry
After you schedule your test day, dive into your MTEL Chemistry study plan! Before you crack open a book or start reviewing exam flashcards, take a timed practice test to get a raw baseline of your readiness. As you continue your exam prep, take regular practice tests to track your progress.
Exam Edge practice tests for the MTEL Chemistry exam offer test-takers key benefits, like helping you identify areas where you need further study and practice. These insights into your test performance will empower you to focus your test prep efforts and prioritize the content areas or skills. This can help you use your study time more effectively and make the most of your efforts before you take the actual exam
Practice tests can also help you to develop your test-taking skills. When you take frequent practice exams, you become more familiar with the format of the MTEL Chemistry exam and learn how to pace yourself throughout it. You will also learn how to approach different types of questions and how to eliminate incorrect answers.
Ultimately, Exam Edge practice tests can help you build your confidence and reduce test-taking anxiety as you become a more comfortable and strategic test-taker. Incorporate MTEL Chemistry practice exams into your study plan to set yourself up for success on test day
Check out our resources to learn more about MTEL Chemistry test prep and practice tests.
Step 4: Take the MTEL Chemistry
On the day of the exam, arrive at the test center early to allow plenty of time to check in and get settled at your testing station. You will need to bring at least one valid, government-issued ID with you. Check on the Massachusetts Tests for Educator Licensure (MTEL) website for other requirements, like:
- Additional forms of identification
- Required materials or supplies
- Other recommended or permitted items, such as water or snacks
- Prohibited items
Once you are settled in your seat or at your testing station, take a moment to center yourself and visualize how to ace the MTEL Chemistry exam. Your diligent studying and use of practice tests have prepared you to tackle the exam with confidence. Trust yourself and your exam prep, pace yourself as you have practiced, and have fun showing off what you know!
MTEL Chemistry Exam Prep
Practice tests are a valuable tool for helping you prepare for the MTEL Chemistry exam. At Exam Edge, we focus on making our clients' career dreams come true by offering world-class practice tests designed to cover the same topics and content areas tested on the actual MTEL Chemistry. Our practice tests provide a realistic simulation of the actual exam, allowing you to become familiar with the format, style, and types of questions you will encounter on the actual test.
Location Information and Website
For more information on scheduling the Massachusetts Tests for Educator Licensure exam, visit our Massachusetts Tests for Educator Licensure (MTEL) information page.
MTEL Chemistry Aliases Test Name
Here is a list of alternative names used for this exam.
- MTEL Chemistry
- MTEL Chemistry test
- MTEL Chemistry Certification Test
- MTEL
- MTEL 67
- 67 test
- MTEL Chemistry (67)
- Chemistry certification